Alzheimer’s wonder drug blocked for use on NHS after officials ignore costs to families
NHS patients will be denied a wonder drug for Alzheimer’s after officials disregarded the costs borne by families.
The National Institute for Health and Care Excellence (Nice) – the quango that decides which treatments are provided on the health service – has deemed that lecanemab is not good value for money.
However, The Telegraph can reveal that the assessment failed to take into account the costs of the disease to families and society.
The rejection by Nice, in draft guidance, came after the drug was given the green light by safety regulators for patients in the early stages of the disease. It is the first ever treatment that slows the progression of Alzheimer’s.
Charities accused Nice of “a fundamental injustice” in disregarding the value of care given by thousands of families. Alzheimer’s Research UK pleaded with Wes Streeting, the Health Secretary, to intervene, saying the situation was “heartbreaking”.
The charity warned that the costs to society would only become more “eye-watering” if the body did not change its stance and allow ground-breaking medication to be funded on the NHS.
The vast majority of care for dementia patients is either provided by loved ones or paid for privately.
Click here to view this content.
In their submission to Nice, manufacturers set out estimates for the “non-medical” costs of care. But Nice’s committee excluded the evidence on the grounds that most of the care was either paid for by families or provided by them unpaid.
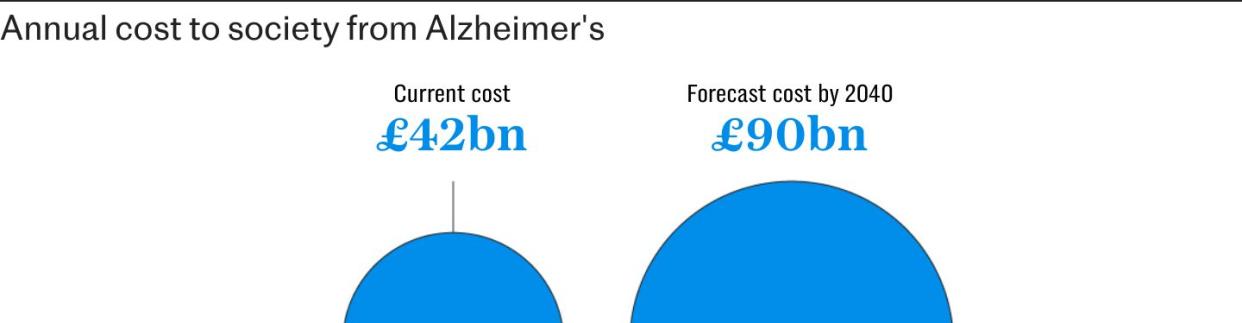
Estimates suggest that the UK spends approximately £42 billion a year on dementia, with most of the costs borne by families and social care bodies. Forecasts have said that this figure could reach £90 billion by 2040.
David Thomas, the head of policy at Alzheimer’s Research, said: “The failure to include the cost of caring in the model is fundamentally unjust. The way these assessments are being carried out is just not fit for purpose”.
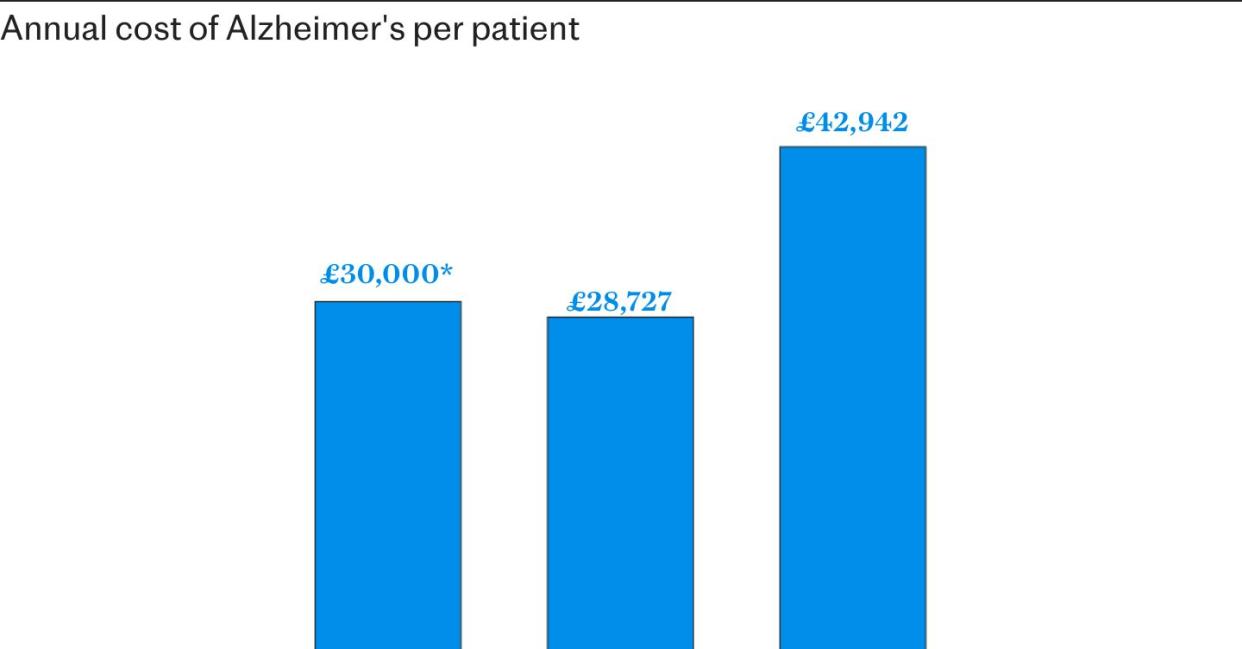
The cost of funding the medication for the 70,000 people for whom the drug has been licensed would have amounted to around £2 billion, estimates suggest.
The figure is almost the same as the existing costs for this group when social care, impact on the economy, healthcare and unpaid help is considered. By the time such patients reach a moderate stage of Alzheimer’s, which lecanemab could delay, annual costs reach £3 billion.
Experts said the decision by Nice would heap more pressure on families and on a social care system that is “on its knees”, with scientists urging Nice to be more “forward-thinking” in approaching a new class of medicine.
The Alzheimer’s Society said families across the country had been left in “uncertainty and confusion” by the rejection of the drug, manufactured by the Japanese pharmaceutical company Eisai in partnership with Biogen.
Lecanemab had been hailed as “the beginning of the end” for Alzheimer’s after trials found that it slowed cognitive decline by 27 per cent in sufferers.
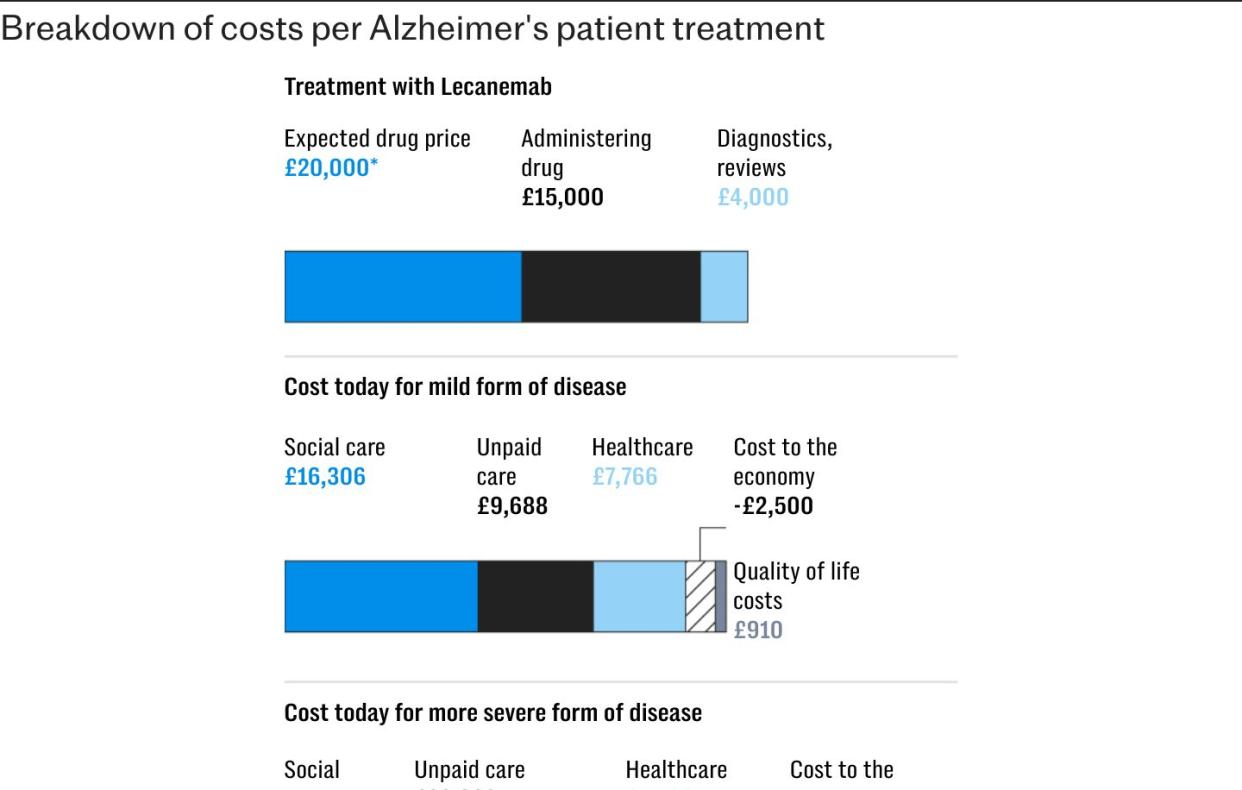
But in draft guidance, Nice said the benefits of the drug were too small to justify its costs. Lecanemab was approved for use in the US last year at an annual cost of $26,500 (£20,000) per patient.

Most of the details of Nice’s costings were shrouded in mystery, with vast amounts of information deemed commercially sensitive.
It said it expected costs of diagnoses, administering the injections and monitoring patients to amount to about £19,000 annually.
While the official price of the drug is expected to be around £20,000, a discount would have been applied for use across the NHS. Independent experts had suggested the combined costs would probably have been more than £30,000 per patient annually.
Nice said the costs were “considerably above the range normally considered cost effective for routine NHS use”. A spokesman confirmed that this range was between £20,000 and £30,000 per year.
Private medical firms are now scrambling to open clinics that can offer the drug, with some expected to charge patients more than £50,000 annually.
Prof Jonathan Benger, the chief medical officer for Nice, said the organisation was “immensely disappointed that we are unable to recommend the treatment in draft guidance today”.
He said lecanemab was one of a new class of drugs, with 27 other medicines in this class also seeking approval by 2030.
Prof Benger said it was “unfortunate and very disappointing” that the costs could not be justified, saying: “The benefits are very small for this drug, the costs are very high”.
Nice’s committee had concluded that lecanemab could slow progression of the disease by between four and six months, with experts saying this could mean “more time socialising, driving and being independent”.
The draft guidance will now go to public consultation until Sept 20.
Prof John Hardy, the chairman of molecular biology of neurological disease at the UCL Institute of Neurology and one of the world’s leading researchers in the field, urged Nice to reconsider.
He said he hoped real-world evidence from the US about the long-term gains from the drug could convince Nice’s committee to change their minds.
The body, which rations the use of medicines by the NHS, has previously faced a backlash over its treatment of patients with Alzheimer’s.
In 2007, it said three drugs that helped with symptoms of the disease should only be restricted to the most severe cases. One of the drugs, Aricept, cost just £2.80 per patient per day.
The decisions were reversed in 2010 after a long campaign that culminated in court proceedings.
Click here to view this content.
Nice said the independent committee did consider the benefit to society, in that it considered quality of life improvements for carers. It said this had “minimal impact” on the balance of costs and benefits, because the benefits were small in relation to costs.
Helen Knight, the director of medicines evaluation at Nice, said: “For Nice to be able to approve a medicine for use in the NHS it must not only provide benefits to patients but it must also represent a good use of NHS resources and taxpayers’ money.
“Lecanemab provides on average four to six months slowing in the rate of progression from mild to moderate Alzheimer’s disease, but this is just not enough benefit to justify the additional cost to the NHS.”
Prof Sir Stephen Powis, the NHS’s national medical director, said: “Lecanemab is the first disease-modifying treatment for Alzheimer’s disease with a market approval in the UK.
“And, to ensure the health system is prepared for future advances in treatments, a dedicated NHS team is also looking ahead to 27 other drugs which are currently in advanced clinical trials that could be potentially approved by 2030.”
A Department of Health and Social Care spokesman said: “It is right that these decisions are taken independently, based on an assessment of the available evidence on the relative costs and benefits of a treatment.
“The Government is committed to continuing to expand research and innovation in this area, with advanced clinical trials for other drugs under way. We will continue to work with NHS England and Nice to make treatments available as and when they’re ready.”


